Evaporation, crystallization, thermal thickening and drying of liquids in one apparatus
Isolation of valuable substances from a liquid
In many cases, the remaining solid is the valuable material that you want to isolate by evaporation and vacuum drying. Sometimes it's the other way around: The evaporating medium is then the valuable substance, which is condensed into a liquid by cooling.
In general, thermal drying in a vacuum mixer dryer only makes economic sense if the mixing goods have been mechanically dehumidified beforehand. Suction filters, chamber filter presses, vacuum membrane filters or centrifuges, for example, are used for mechanical dehumidification. Some liquids are both a solution and a suspension. They cannot be dehumidified mechanically and must be thickened by evaporation.
Sometimes the stirrer in the evaporator causes problems
The discontinuous evaporators are heatable, vacuum-tight containers. Optionally, they can be equipped with heatable elements. For a more effective heat supply, a liquid agitator is installed. Such liquid agitators improve the exchange of material and heat. However, their mixing efficiency decreases as the viscosity of the liquid increases. In some cases, the liquid can even become viscoplastic and sticky, or the product can take on a flow behavior similar to that of chewing gum or moist sand. This can block and damage a liquid agitator. amixon® has developed special SinConvex® and SinConcave® helix mixing tools for stirring/mixing such poorly flowing dispersions.
amixon® mixing tools are equally suitable for solids and liquids. amixon® vacuum mix-dryers can also mix, evaporate, thicken and dry viscoplastic suspensions/liquids.
Cost advantage: Three processes in one amixon® device
The processing of toxic or expensive substances requires containment solutions to ensure that the substances cannot escape from the system. The smaller and more compact such a system is built, the better. It is also advantageous if as many process steps as possible can be carried out in one system. Such a multi-purpose system is also advantageous, for example, if the substances arehighly corrosive or abrasive when wet. In both cases, the aim is to carry out as many process steps as possible in a closed, compact system.
It then makes sense to use a vacuum mixer dryer for both evaporation and drying. In order to operate an evaporation process economically, the vapor pressure values are required. These depend on the temperature and the concentration curve.
Determination of the vapor pressure
The vapor pressures of a complex liquid containing salts and finely dispersed solids are determined below. The aim of the process is to isolate the solids and the liquid components contained in a complex suspension/solution. The components are: Water, iron acetate, calcium chloride, calcium permanganate, chromium, sodium carbonate, sodium chloride, sodium fluoride, sodium hydrogen phosphate, sodium hydroxide, sodium nitrate, sodium sulphate, silicon dioxide, metal soaps, ...
The isoteniscope method
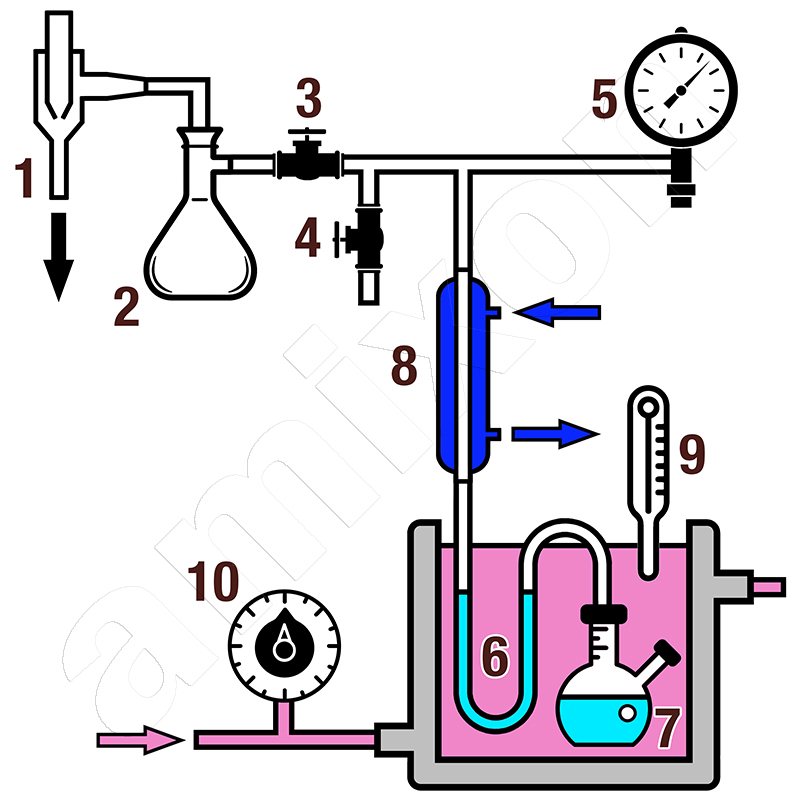
Automated systems for determining vapor pressure may already be available today. Nevertheless, it is interesting to look at an experimental setup. Here the vapor pressure is determined using an isoteniscope vessel. This test setup allows the vapor pressures to be determined accurately even at absolute pressures of only a few mbar.
The liquid/suspension to be analyzed is located in the siphon and in the sample vessel. The liquid serves as a barrier medium. Both the siphon and the vessel are located in the temperature control vessel, which is easily visible. Valves (a) and (b) are used to adjust the vacuum so that the two minisinks in the siphon are at the same level. The system pressure set in this way is the respective vapor pressure of the liquid. The values change when the temperature of the suspension changes. The values also change if the concentration of the suspension changes.
Comparison between distilled water and the suspension
The respective measured values are determined by adjusting pressure gauges 1 and 2. A Woulff bottle is installed upstream of the vacuum pump to stabilize the applied vacuum. The cooling coil above the isoteniscope vessel is used to recondense the sealing medium.
Calculation of the vapor pressure curve
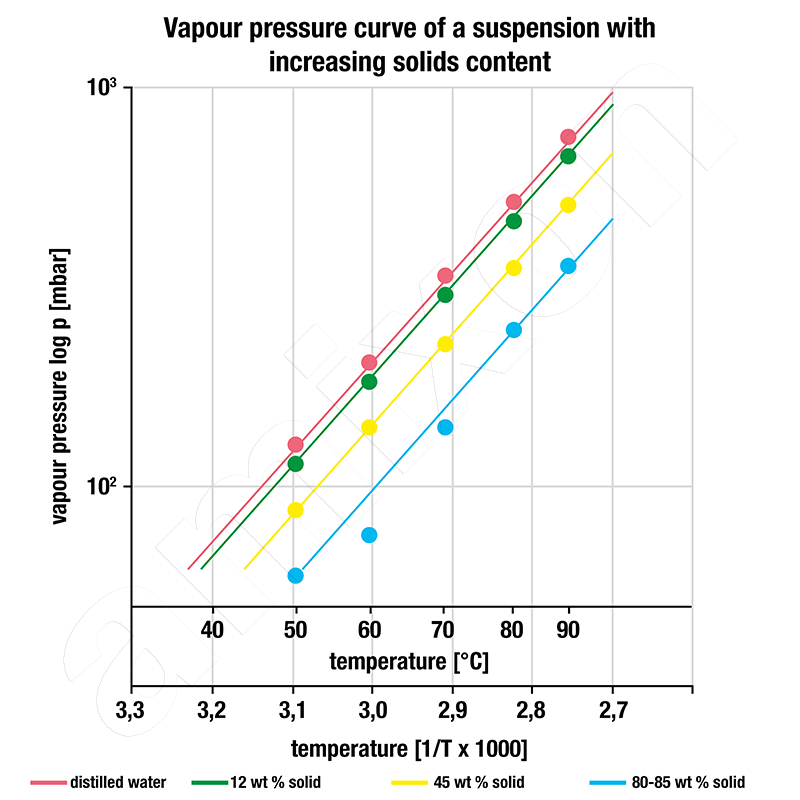
Clausius Clapeyron's simplified formula describes the dependence of the vapor pressure of a liquid on the temperature as a modified equation of degrees. A suitable diagram was used for the graphical evaluation of the experiments, which clearly illustrates the Clausius-Clapeyron formula. The temperature in Kelvin is plotted on the abscissa as a reciprocal value (multiplied by 1000). If the pressure is plotted in logarithmic form on the ordinate, the vapor pressures of the liquids appear as parallel degrees. The gradient of the degrees is a measure of the enthalpy of the liquid.
Three interesting observations can be made on the basis of the diagram:
- How many different liquids are contained in the suspension? In this example, the relatively straight course of the measuring points shows that the liquid phase of this suspension only consists of a single liquid.
- What type of liquid is it? Since the slope of the straight line is the same as that of water, regardless of the concentration, it is most likely water.
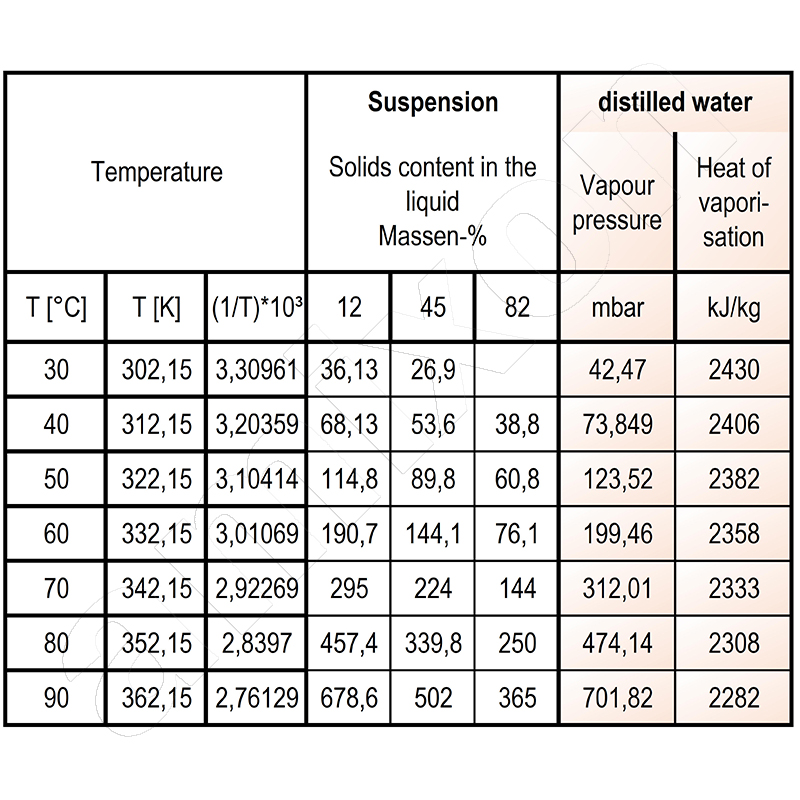
- At what solids concentration does the transition from evaporation to vacuum drying take place? You can see how the vapor pressure decreases as the concentration of the suspension increases. The 12% suspension evaporates at 70°C when the system pressure is 295 mbar. During evaporation, the pressure must be reduced to 144 mbar. Evaporation ends at around 80 to 85% solids content by mass. The drying of the wet solid dispersion then begins.
In this case, the evaporation energy to be applied is easy to determine. The amount of heat to be supplied is similar to the heat of vaporization of water at 70°C: around 2333 kJ/kg. This initially ignores the fact that heat is lost through radiation.
Evaporation, thickening, vacuum mix-drying with high efficiency
Both batch evaporators and vacuum mix-dryers have good efficiencies when they are operated at high filling levels. In this case, the amixon® mixing dryer is repeatedly filled with the suspension during evaporation as soon as the level falls below a certain level. This happens in many steps or continuously. The concentration of solids continues to increase. It is therefore a continuous evaporation process. It only ends when the maximum filling level of the appliance has been reached. The final drying of the solids also takes place at a high filling level.
This means that evaporation and drying take place conveniently in one compact apparatus. The same apparatus can also be used to cool the dry material. The batch of solids is then filled into sacks, big bags or bulk containers.
The design of the peripheral system components such as the vapor filter, condenser, heat generator and the dimensioning of the agitator/mixer drive will be covered in further blog posts at a later date.
Piloting From the lab to technical scale
amixon® has more than 30 test machines in its technical center available. Many of them are excellent vacuum mixers/ evaporators/ dryers. We cordially invite you. Come to our test center with your products and test the following processes:
- Mixing / blending
- Evaporating,
- Thickening,
- Vacuum drying,
- Wetting,
- Agglomerate,
- Disagglomerate,
- Coating
- Drying,
- Calcination or for
- Carrying out active ingredient syntheses.
© Copyright by amixon GmbH