Diffusion and doping for nanostructured materials
Homogenization through mechanically generated change of place
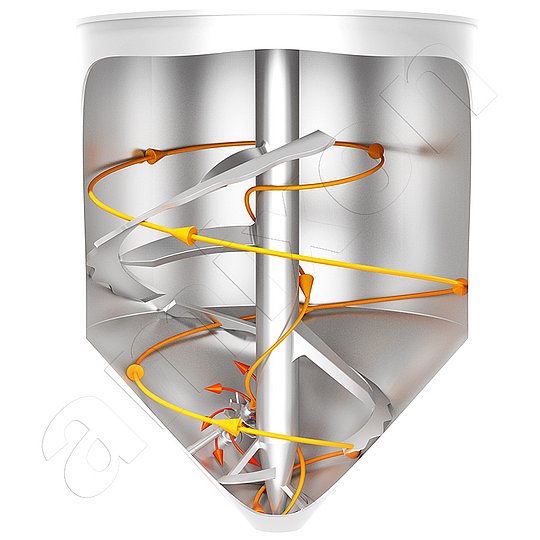
This blog post focuses on the question of how a mixer can modify powdered materials by diffusion. The powders can be dry, moist or suspended. amixon® process equipment is designed for high pressures and temperatures. This supports diffusion.
In dynamically operating mixing machines, substances are homogenized by flowing and shearing through mixing tools. The mixing tasks can vary in difficulty, depending on the type and flowability of the mixing components. The dead-space-free mixing of nanodisperse bulk materials is challenging, as the rheological properties are similar to those of sticky wet materials.
Differences in concentration are compensated by diffusion.
Although the gases appear to be stationary in a closed vessel, the gas molecules perform minimal zigzag motions against each other and energize the gas molecules in their neighborhood. The strength of the zigzag movements and the pulse exchange depends on the prevailing temperature. These micro-movements of the molecules only come to a halt if the gas were to be cooled to absolute zero, i.e. minus 273.15°C.
If there are gases of different density or temperature in a vessel, homogenization takes place automatically. The different gases diffuse into each other. This is due to the high efficiency of gas diffusion. Diffusion is a mass transport. It compensates for concentration differences such as density, temperature and pressure.
Liquids of different densities also mix automatically by diffusion, but this homogenization process takes much longer than for gases.
It takes even longer when solids diffuse into each other.
Although the gases appear to be stationary in a closed vessel, the gas molecules perform minimal zigzag motions against each other and energize the gas molecules in their neighborhood. The strength of the zigzag movements and the pulse exchange depends on the prevailing temperature. These micro-movements of the molecules only come to a halt if the gas were to be cooled to absolute zero, i.e. minus 273.15°C.
If there are gases of different density or temperature in a vessel, homogenization takes place automatically. The different gases diffuse into each other. This is due to the high efficiency of gas diffusion. Diffusion is a mass transport. It compensates for concentration differences such as density, temperature and pressure.
Liquids of different densities also mix automatically by diffusion, but this homogenization process takes much longer than for gases.
It takes even longer when solids diffuse into each other.
The speed of diffusion
Adolf Fick, a German physiologist (1829-1921), described the diffusion of substances through a material with two formulas. They state that the diffusion current is proportional to the concentration difference but opposite to the direction of the concentration gradient.
The letter J is often used for the diffusion current density in the technical literature. In the SI-compliant representation, the diffusion current density is measured in (kg/(m²*s)). But also the representation "mol per square meter per second" (mol/(m²*s)) is common.
(1) J = -D * (ΔC/Δx)
in the process is
J: the diffusion flux (mass or molecules per area and time).
D: the diffusion coefficient of the substance in the material (m²/s)
ΔC: the difference in concentration of the substance over the distance ΔC/Δx: Concentration gradient
The second law takes into account that both the diffusion coefficient and the concentration gradient change with time due to the spatial distribution of the concentration.
(2) ∂C/∂t = D * ∇²C
in the process is
∂C/∂t: the change in concentration over time.
∇²c: the Laplace operatorforthespatial change ofthe concentration
The diffusion coefficient D can also be calculated.
D = D0 * e (-Q/(R * T))
in the process is
D0: Diffusion constant [m²/s] is a material-specific constant
Q: Activation energy [kg * m2/s-2]
R: general gas constant [J/(kg * K)].
T: Temperature [K]
Very slow mass transfer during solid diffusion: The diffusion coefficient has approximately a value of 10-5 m2/s for gases, 10-10 m2/s for liquids and
10-20 m2/s for solids.
Adolf Fick, a German physiologist (1829-1921), described the diffusion of substances through a material with two formulas. They state that the diffusion current is proportional to the concentration difference but opposite to the direction of the concentration gradient.
The letter J is often used for the diffusion current density in the technical literature. In the SI-compliant representation, the diffusion current density is measured in (kg/(m²*s)). But also the representation "mol per square meter per second" (mol/(m²*s)) is common.
(1) J = -D * (ΔC/Δx)
in the process is
J: the diffusion flux (mass or molecules per area and time).
D: the diffusion coefficient of the substance in the material (m²/s)
ΔC: the difference in concentration of the substance over the distance ΔC/Δx: Concentration gradient
The second law takes into account that both the diffusion coefficient and the concentration gradient change with time due to the spatial distribution of the concentration.
(2) ∂C/∂t = D * ∇²C
in the process is
∂C/∂t: the change in concentration over time.
∇²c: the Laplace operatorforthespatial change ofthe concentration
The diffusion coefficient D can also be calculated.
D = D0 * e (-Q/(R * T))
in the process is
D0: Diffusion constant [m²/s] is a material-specific constant
Q: Activation energy [kg * m2/s-2]
R: general gas constant [J/(kg * K)].
T: Temperature [K]
Very slow mass transfer during solid diffusion: The diffusion coefficient has approximately a value of 10-5 m2/s for gases, 10-10 m2/s for liquids and
10-20 m2/s for solids.
Efficient forms of diffusion in technical ceramics and powder metallurgy
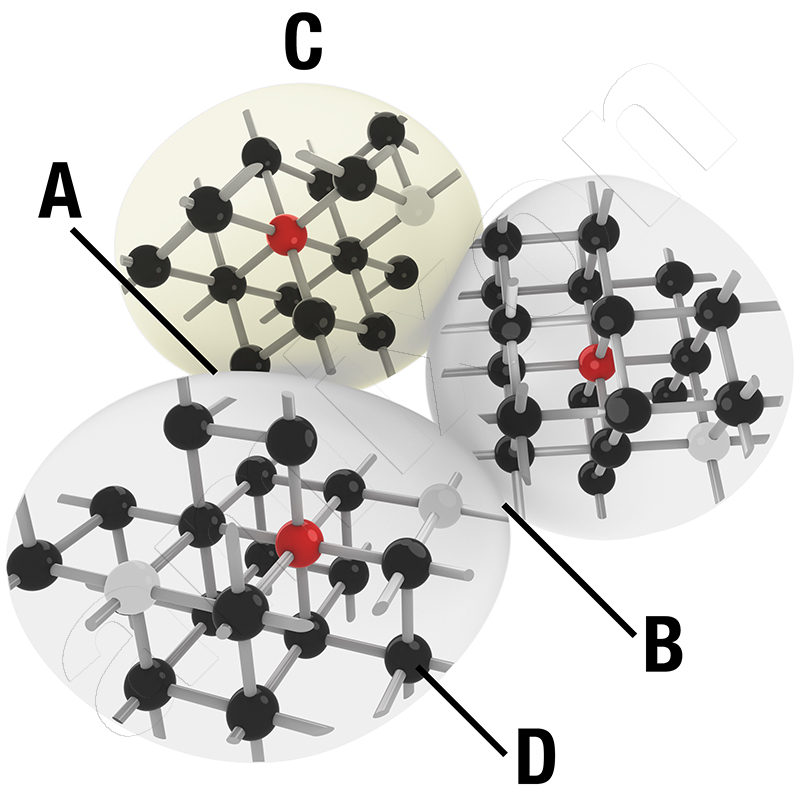
Diffusion plays an important role in powder metallurgy when foreign materials in finely dispersed form are pressed into a shaped body. In this case, one speaks of a "mechanical alloy".
So-called foreign diffusion takes place at the phase boundaries, grain boundaries and surfaces of the microstructure. Atomic groups from regions of higher potential slowly migrate to regions of lower potential. These processes are more intense the smaller the microstructural particles involved are and the more they are pressed against each other.
Diffusion can be a suitable method for doping high-performance materials by introducing foreign atoms into disrupted lattice structures. Diffusion is particularly strong when concentration gradients meet in the form of nanoscale powders. This is particularly the case at phase and grain boundaries.
Diffusion is particularly strong,
- when the goods are present as nanofine dispersions.
- if there is as large a concentration gradient as possible.
- when the process temperatures are high.
- when particles with high concentration meet particles with low concentration as often as possible.
- when the particles slide closely along each other - without being surrounded by a gaseous phase.
Diffusion plays an important role in powder metallurgy when foreign materials in finely dispersed form are pressed into a shaped body. In this case, one speaks of a "mechanical alloy".
So-called foreign diffusion takes place at the phase boundaries, grain boundaries and surfaces of the microstructure. Atomic groups from regions of higher potential slowly migrate to regions of lower potential. These processes are more intense the smaller the microstructural particles involved are and the more they are pressed against each other.
Diffusion can be a suitable method for doping high-performance materials by introducing foreign atoms into disrupted lattice structures. Diffusion is particularly strong when concentration gradients meet in the form of nanoscale powders. This is particularly the case at phase and grain boundaries.
Diffusion is particularly strong,
- when the goods are present as nanofine dispersions.
- if there is as large a concentration gradient as possible.
- when the process temperatures are high.
- when particles with high concentration meet particles with low concentration as often as possible.
- when the particles slide closely along each other - without being surrounded by a gaseous phase.
Accelerated diffusion in the amixon® reactor
to 1)
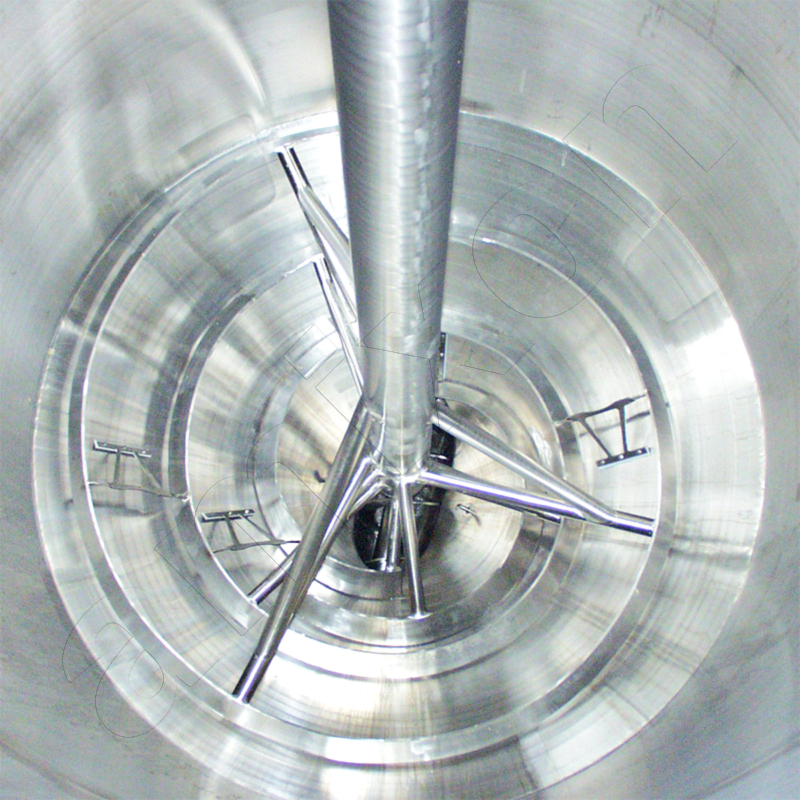
The mixing process in the amixon® reactor is always free of dead space and efficient - regardless of the speed of the mixing tool. The flow properties of the mixing goods - dry, moist, wet or suspended - are irrelevant. In addition, the precision mixing takes place regardless of the filling level.
to 2)
In the amixon® reactor, the components can be in any aggregate state to diffuse into each other: dry, liquid or gaseous.
to 3)
amixon® reactors can run predetermined heating/cooling curves with an accuracy of a few tenths of a Kelvin. Up to 350 °C. Current further developments of amixon® even target temperatures above 600°C.
to 4)
The dead space free mixing process ensures that particles/liquids or gases are in constant and random contact with each other. An amixon® test reactor operates with up to 25 bar overpressure. (amixon® process plants meet the legal requirements of TA Luft. They are technically tight - even at high temperatures and pressures).
to 5)
Solid-solid reaction / diffusion: In the amixon® apparatus, a steep concentration gradient can be generated by applying a high vacuum. In single-phase mixtures (without gas phase), the solid particles rub closely together - even at high temperatures.
to 1)
The mixing process in the amixon® reactor is always free of dead space and efficient - regardless of the speed of the mixing tool. The flow properties of the mixing goods - dry, moist, wet or suspended - are irrelevant. In addition, the precision mixing takes place regardless of the filling level.
to 2)
In the amixon® reactor, the components can be in any aggregate state to diffuse into each other: dry, liquid or gaseous.
to 3)
amixon® reactors can run predetermined heating/cooling curves with an accuracy of a few tenths of a Kelvin. Up to 350 °C. Current further developments of amixon® even target temperatures above 600°C.
to 4)
The dead space free mixing process ensures that particles/liquids or gases are in constant and random contact with each other. An amixon® test reactor operates with up to 25 bar overpressure. (amixon® process plants meet the legal requirements of TA Luft. They are technically tight - even at high temperatures and pressures).
to 5)
Solid-solid reaction / diffusion: In the amixon® apparatus, a steep concentration gradient can be generated by applying a high vacuum. In single-phase mixtures (without gas phase), the solid particles rub closely together - even at high temperatures.
Please visit us in our test center. We cordially invite you to carry out trials with your original products.
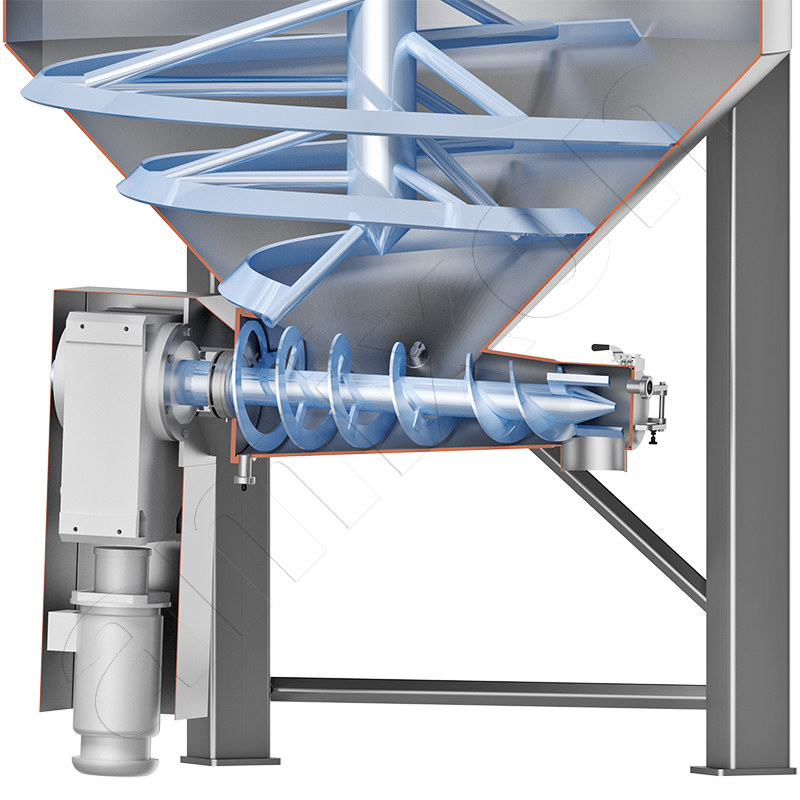
Gyraton® mixer for the homogenisation of large batches.
High-performance materials are usually processed in small batches. If you consider, for example, the manufacture of batteries, wind turbines, components for energy transmission or communications technology or seawater desalination plants, these materials are required in ever larger quantities. However, these large masses of material must be homogeneous.
This is where amixon® can make a valuable contribution. Gyraton® mixers can homogenise large quantities of bulk material (70 m³) in batches. However, the same Gyraton® mixer can also mix continuously. In either case, fluctuations in quality can be equalised.
Gyraton® mixers mix without dead space, homogeneously and extremely gently - regardless of the bulk material properties. The drive power of a Gyraton® mixer is very low. The products can be nanodispersed, agglomerated, dry, moist or wet.
High-performance materials are usually processed in small batches. If you consider, for example, the manufacture of batteries, wind turbines, components for energy transmission or communications technology or seawater desalination plants, these materials are required in ever larger quantities. However, these large masses of material must be homogeneous.
This is where amixon® can make a valuable contribution. Gyraton® mixers can homogenise large quantities of bulk material (70 m³) in batches. However, the same Gyraton® mixer can also mix continuously. In either case, fluctuations in quality can be equalised.
Gyraton® mixers mix without dead space, homogeneously and extremely gently - regardless of the bulk material properties. The drive power of a Gyraton® mixer is very low. The products can be nanodispersed, agglomerated, dry, moist or wet.
© Copyright by amixon GmbH