Concentration gradient
When substances move without mechanical conveyance, the concentration gradient acts as a driving force. For example, for diffusion or heat transfer.
They also play an important role in food and pharmaceutical technology. Instant products dissolve quickly as the pore structures favour transport. Tablets release active ingredients via diffusion along a concentration gradient.
In mass transfer, they are the driving force behind diffusion. During drying, for example, water diffuses from the inside of particles to the outside. In gas scrubbing, gas molecules migrate into a solvent. In extraction, for example of caffeine from coffee beans, transport also takes place along a concentration gradient.
In separation technology, differences in concentration are the driving force behind many processes. In membrane systems such as reverse osmosis or dialysis, the material flows through the membrane along a gradient.
Concentration gradients also play a driving role in environmental technology. Pollutants move through soils and pore spaces along their concentration gradients. In fuel cells, gradients determine the transport of reactants and products.
A concentration gradient describes the spatial change in the concentration of a substance. It indicates how much the concentration c changes per unit length x.
Mathematically, it is defined as a vector that is differentiated according to the path.
dc/dx = ∇c = (∂x/∂c, ∂y/∂c, ∂z/∂c)
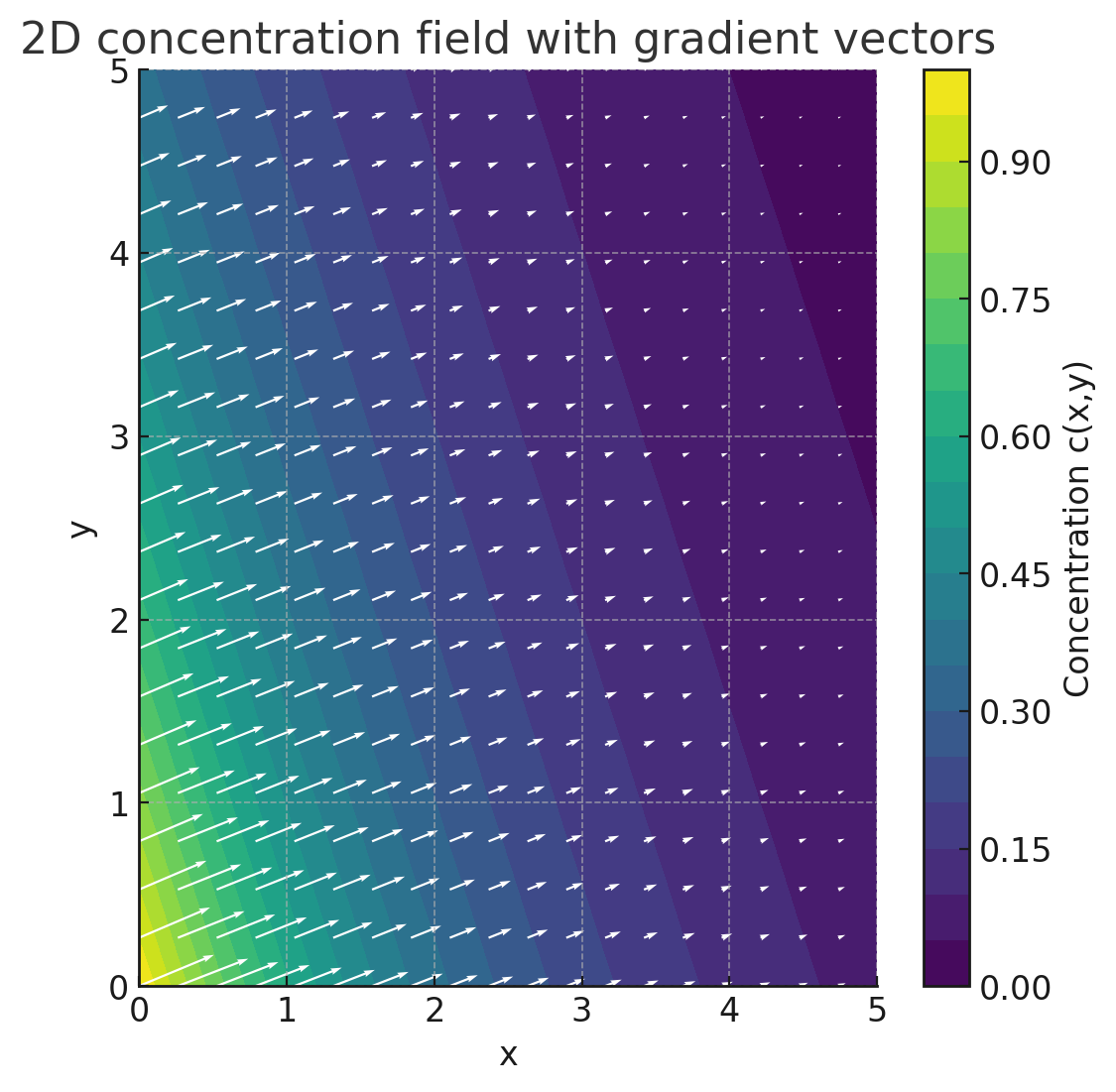
One possibility for two-dimensional vectorial representation is shown on the left. The length of the vectors shows the concentration gradient. The direction of the arrows of the vectors indicates the direction of the desired diffusion at the respective location.

