solid-liquid separation
Solid-liquid separation is used to separate dispersed solids from liquids or vice versa. A basic distinction is made between mechanical and thermal separation processes. Mechanical processes exploit differences in density, particle size or filterability, while thermal processes are based on the evaporation of the liquid.
Mechanical solid-liquid separations are energy-efficient, as the aggregate state of the liquid remains unchanged. They are preferred when large quantities of liquid are processed and a residual moisture content can be tolerated. It is not possible to achieve completely dry solids using mechanical methods alone. Therefore, a thermal drying step often follows, for example, vacuum mixing drying, roller drying, spin drying, thin-film drying or spray drying.
If the solid is dissolved in the liquid, it can only be separated using thermal processes such as evaporation, crystallisation and drying. These processes also belong to solid-liquid separation, as they aim to completely remove the liquid phase, but are achieved by supplying energy and changing the phase of the liquid.
Typical devices for mechanical dewatering are filter presses, belt filters, decanter or screw centrifuges, and vacuum filters. The selection of the separation unit depends on the sludge characteristics, the particle size distribution, the viscosity of the liquid, and the desired dry matter content.
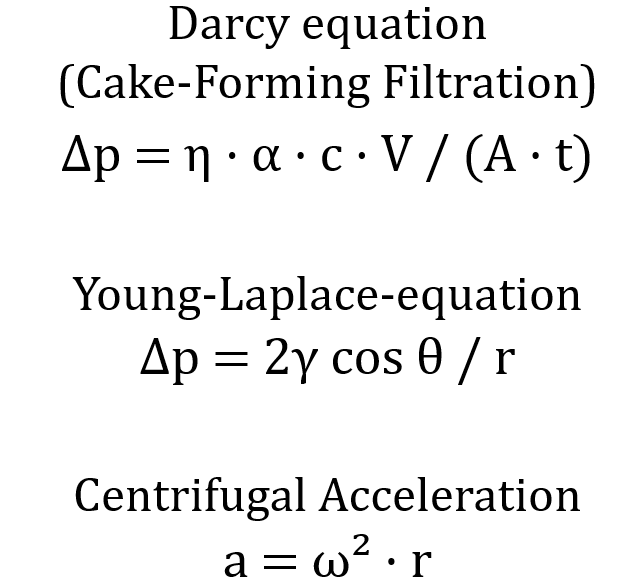
During filtration, a filter medium is flowed through, which retains the solid particles. In the simplest case, Darcy's law applies to cake-forming filtration:
Δp = η * α * c * V / (A * t)
Here, Δp stands for the pressure difference, η for the viscosity of the liquid, α for the specific filter resistance, c for the solids concentration, V for the filtrate volume, A for the filter area and t for the time. It describes the relationship between the filtrate volume, pressure difference and filtration time.
In addition to viscosity, surface tension also influences mechanical solid-liquid separation. High surface tension makes it more difficult for the liquid to escape from fine pores, as it leads to stronger capillary bonding. Reduced surface tension – for example, through surfactants – improves drainage and reduces residual moisture. This relationship can be described using the Young-Laplace equation.
Δp = 2γ cos θ / r
Here, Δp is the capillary pressure, γ is the surface tension, θ is the wetting angle and r is the pore radius. Centrifugation utilises separation by centrifugal forces. The effective acceleration factor a is calculated as follows:
a = ω² * r
where ω = angular velocity and r = radius of the centrifuge. The higher the centrifugal acceleration, the better the liquid residues can be spun off.
In wastewater treatment, solid-liquid separation is used to dewater sewage sludge in order to reduce transport and disposal costs. Before mechanical separation, the suspensions are often conditioned. This is achieved by sedimentation, flocculation, flotation, crystallisation or chemical precipitation.
In chemical and pharmaceutical process engineering, a large number of syntheses end with precipitation or crystallisation of the target product. Continuous-operation decanter centrifuges, pressure filters or vacuum filter presses are used for this purpose. In the subsequent thermal step, the filter cake is dried to remove any remaining moisture.
The process concludes with vacuum mixing and drying. This type of drying is called contact drying. It enables gentle final drying at low temperatures and reduces product damage caused by localised overheating.
The vacuum mixing dryers from amixon® are characterised by large specific heat transfer surfaces and gentle, three-dimensional mixing. This allows sensitive powders to be dried with minimal thermal and mechanical stress. In any case, users should carry out drying tests with the machine manufacturers.

