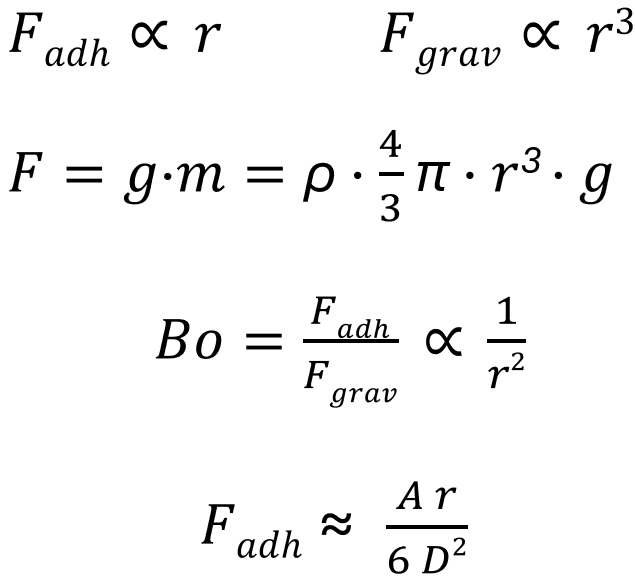
Intensive mixing
Nanodisperse bulk solids do not flow, they stick together. They tend to form clumps. This is particularly critical with very small particles, such as those in the nano or submicron range. In this case, interparticle adhesive forces dominate the behaviour of the material. The smaller the particle radius r, the more dominant the adhesive forces Fadh are. For the weight force F or Fgrav, the following applies in the case of spherical particles:
Fgrav = g · m = ρ · 4/3 · π · r3 · g
m: weight force
g: gravitational acceleration
ρ: Density of the solid
r: Idealised radius of the particles
These adhesive forces are Van der Waals forces, electrostatic attraction or capillary forces. They often exceed the gravitational force Fgrav of the particles by a thousand times. The force ratios can be clearly expressed using the Bond number ‘Bo’:
Bo = Fadh/Fgrav ∝ 1/r2
Where Fadh is the adhesive force between particles and Fgrav is their gravitational force. For nanoparticles, the following applies if the particles are spherical:
Fadh ∝ r Fgrav ∝ r3
The result is severely restricted flow behaviour. The particles adhere to each other, form micro-agglomerates and are difficult to process. High shear forces are required to break up these agglomerates. This can be achieved, for example, with air jet mills, shear dispersers or high-performance mixers.
amixon® offers two solutions:
- High-shear blades
- Rotor-stator systems
Both systems generate very high shear gradients locally through impact and friction effects. This breaks up agglomerates and separates primary particles. It is critical that the particles do not immediately clump together again. They should therefore be quickly coated with other particles of similar size from the mixture. It is sometimes advisable to add suitable liquids to prevent re-agglomeration. These must also be efficiently mixed into the powder.
Precision mixers from amixon® are particularly well suited for such processes because they meet two key requirements.
- High mixing quality for the entire batch, regardless of whether the filling level of the mixer is small (100 litres) or large (several cubic metres).
- Efficient micro-dispersion: Thanks to its high energy density, the mixer can reliably separate even the finest agglomerates.
Explanation of the size of the adhesive force between small particles:
Fadh ≈ A·r/(6·D2)
A: Hamaker constant (material property)
D: Distance between the particles
r: Particle radius
The adhesive force between two particles increases linearly with the particle radius r. It increases inversely proportional to the square of the distance D between the particle surfaces:
Fadh ∝ r/D2
This means that the closer the particles are to each other, the stronger they adhere to each other. In a compacted powder bed, the particle distances D become very small. This causes the adhesive force to increase massively. This also explains the high strength of components made of sintered metal and sintered engineering ceramics.

