Isoteniscope method
The isoteniscope method is a physical-chemical measuring method for determining the vapour pressure of a liquid as a function of temperature. It is one of the static methods of vapour pressure determination and is used in particular in thermodynamics and in the process engineering design of evaporation processes.
In processing technology, the evaporation of liquids is a key process step, for example for concentration, crystallisation or the separation of temperature-sensitive substances. Another example is food or pharmaceutical production, where heat-sensitive components need to be separated gently. Another area of application is the recovery of solvents from residues during rectification.
The efficiency and thermal load of the product depend crucially on the boiling behaviour of the liquid. This in turn is influenced by the vapour pressure, which changes with the temperature.
The vapour pressure defines the pressure at which the liquid is in equilibrium with its own vapour phase. As the temperature increases, the vapour pressure rises and the boiling point shifts under a given ambient pressure. In technical systems, especially in vacuum conditions or multi-stage evaporation systems, precise knowledge of the vapour pressure curve is therefore essential.
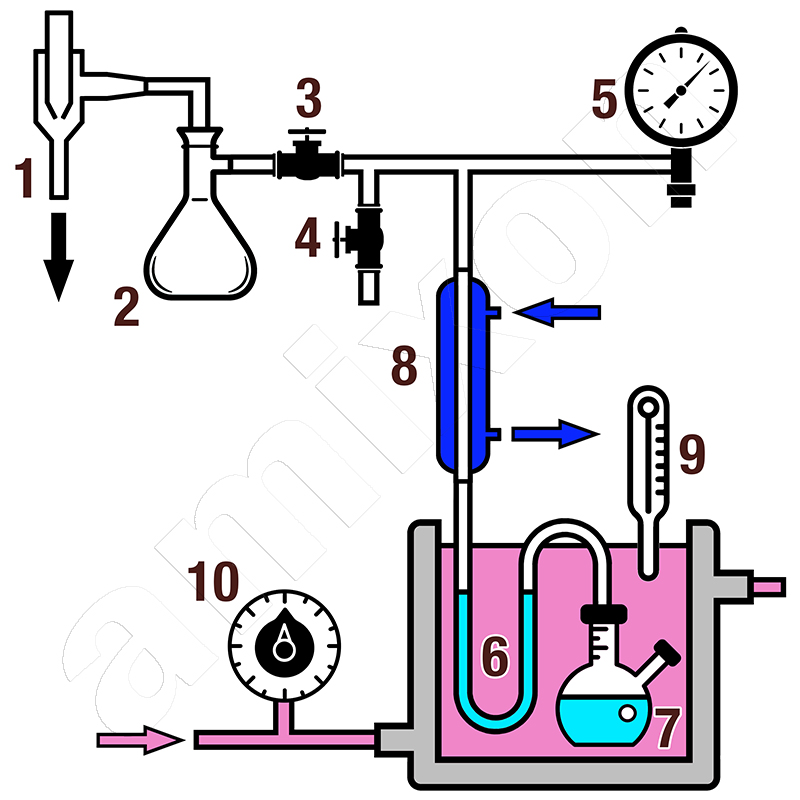
An isoteniscope is a simple but precise measuring device made of glass. It consists of a U-shaped tube system that is partially filled with the liquid to be analysed and a reference liquid (often mercury). The sample is heated in a thermostated bath while the pressure in the system is controlled by a vacuum or a known gas atmosphere. The equilibrium pressure of the liquid can then be determined by the difference in height of the mercury columns. The measured value corresponds to the equilibrium vapour pressure of the sample at the respective temperature. Repeated measurements at different temperatures result in a vapour pressure curve. On the basis of this curve, thermodynamic variables such as the enthalpy of vaporisation can be determined by linearisation according to the Clausius-Clapeyron equation.
ln(p) = −ΔHvap /(R · T) + C
p : Vapour pressure
ΔHvap : Enthalpy of vaporisation
R : Universal gas constant
T : absolute temperature
C : Integration constant
Vacuum mixing dryers and evaporation plants are available in the amixon technical centre. They can be used for process testing and product development.

