Surface protection
Particularly high requirements apply in the maritime environment, for example in shipping, offshore energy generation or coastal installations. Here, chlorides, changing moisture levels, UV radiation and mechanical stresses all have an effect.
Effective surface protection is also crucial in chemical engineering. Even high-alloy materials can be attacked under unfavourable conditions. This also applies to Ni-Cr-Mo alloys. Local attacks occur mainly on scratches, edges, stress zones or in contact zones with low-alloy steels.
High-alloy nickel-based materials such as Alloy 59 have very high corrosion resistance. However, in mixed constructions, they can be galvanically coupled. In combination with unalloyed or low-alloy steels, corrosion can accelerate locally. This is particularly true if the protective surface is mechanically damaged. In such cases, the defects must be ground out.
Rubber coatings are often used in highly acidic media. An elastic and chemically resistant coating separates the metal from the medium. Multi-layer systems are particularly effective. These combine a durable hard rubber coating with a softer top layer. This combines chemical resistance and abrasion resistance. Such systems are often used in stirring vessels, mixers and apparatus for solids and suspensions.
A long service life requires a corrosion-resistant design. This includes sufficient wall thicknesses, suitable radii and the finishing of weld seams. Cavities must be avoided.
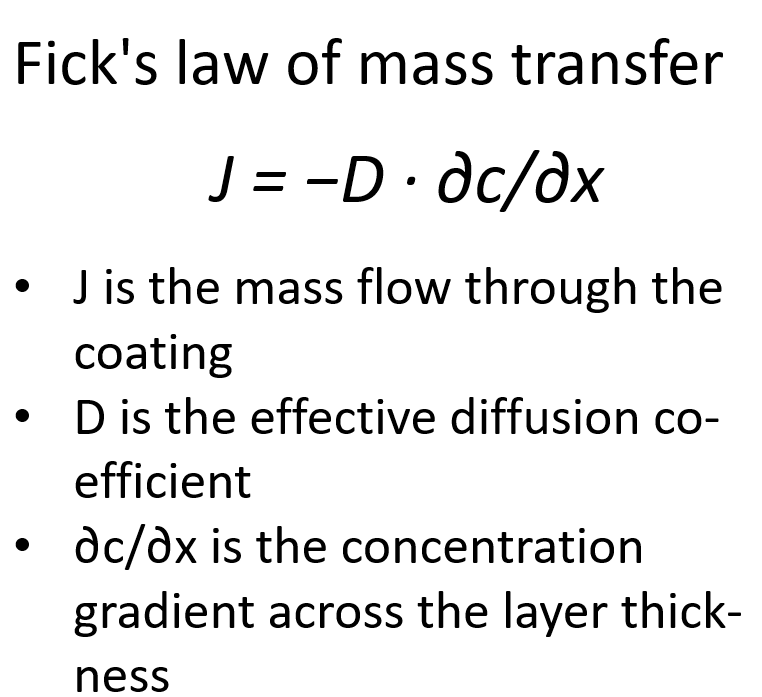
In addition to chemical resistance, water vapour permeability is a key selection criterion for coatings. Some polymer coatings are stable against acids. At the same time, they can be permeable to water vapour or acid components. These diffuse slowly through the layer. This leads to infiltration and blistering. The mass transfer can be described by Fick's first law:
J = −D · ∂c/∂x
- J is the mass flow through the coating
- D is the effective diffusion coefficient
- ∂c/∂x is the concentration gradient across the layer thickness
As the diffusion coefficient increases and the concentration difference grows, the driving force for infiltration increases. Low-diffusion systems are therefore required for aqueous acids and hot media. Minimum layer thicknesses and multi-layer systems are often used.

