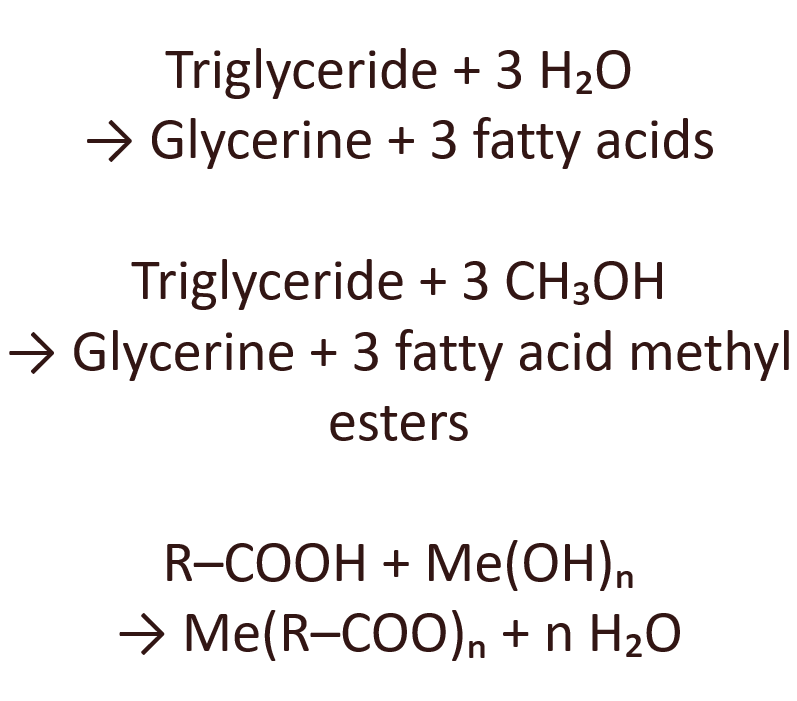
Oleochemistry
Oleochemistry is a branch of the chemical industry that deals with the material use of natural fats and oils. The raw materials used are predominantly vegetable oils such as palm oil, palm kernel oil, rapeseed oil and soybean oil, as well as animal fats. Oleochemical processes replace petrochemical raw materials in many applications.
Fats and oils consist mainly of triglycerides. These are esters of glycerine and long-chain fatty acids. Typical fatty acids are palmitic acid, stearic acid, oleic acid and lauric acid. The chemical composition determines the physical and functional properties of the products.
A central process in oleochemistry is the splitting of triglycerides. This is done by hydrolysis or transesterification. Hydrolysis produces free fatty acids and glycerol. The simplified reaction equation is:
Triglyceride + 3 H₂O → Glycerol + 3 fatty acids
Transesterification with alcohols, usually methanol, produces fatty acid methyl esters and glycerol. This process is the basis for biodiesel production, among other things. The reaction can be written as:
Triglyceride + 3 CH₃OH → Glycerol + 3 fatty acid methyl esters
Metal soaps are metal salts of long-chain fatty acids. They are produced by neutralising free fatty acids with metal hydroxides or metal oxides. A general reaction equation is:
R–COOH + Me(OH)ₙ → Me(R–COO)ₙ + n H₂O
Carbon fatty acid and metal hydroxide produce the metal salt of a fatty acid (metal soap) and water. R stands for the hydrocarbon residue of the fatty acid and Me for a metal such as calcium, magnesium, zinc, aluminium or lithium.
Metal soaps based on palm oil fatty acids are of particular economic importance. Palm oil is available worldwide and has a consistent fatty acid composition. This facilitates reproducible processes and consistent product quality.
Metal soaps have an extremely wide range of applications. They are used as lubricants and greases in plastics processing. They act as stabilisers in PVC. They serve as thickeners in lubricating greases. They are used as release agents, defoamers or water repellents. In paints and varnishes, they influence drying, rheology and surface quality. In construction chemistry, they are used for water repellency. Oleochemical derivatives also play an important role in the cosmetics, pharmaceutical and detergent industries.
The production of metal soaps requires intensive mixing of the reaction partners. Heat transfer, mass transfer and reaction kinetics are closely linked. The reactions can be carried out discontinuously or continuously. Product quality depends heavily on homogeneity, temperature control and residence time.
Industrial mixer reactors are used for chemical reactions. They are exposed to high mechanical and thermal loads. These include rapid pressure-vacuum changes and pronounced temperature changes during process control. Process equipment from amixon® has proven particularly effective under these conditions. It is designed for reactive solid-solid and solid-liquid systems. It wets the solids evenly. They allow controlled and reproducible reaction control and vacuum drying. At the same time, they ensure efficient heat dissipation, even during exothermic reactions.
amixon® mixers are then used to homogenise the end products. The mixing tools operate at low speeds and still achieve short mixing times. This prevents shear forces, dust release and unwanted heating. These properties are valuable because many end products are in powder form. They pose a high risk of explosion and ignition.
Oleochemical products are considered renewable, bio-based and, in many cases, biodegradable.

