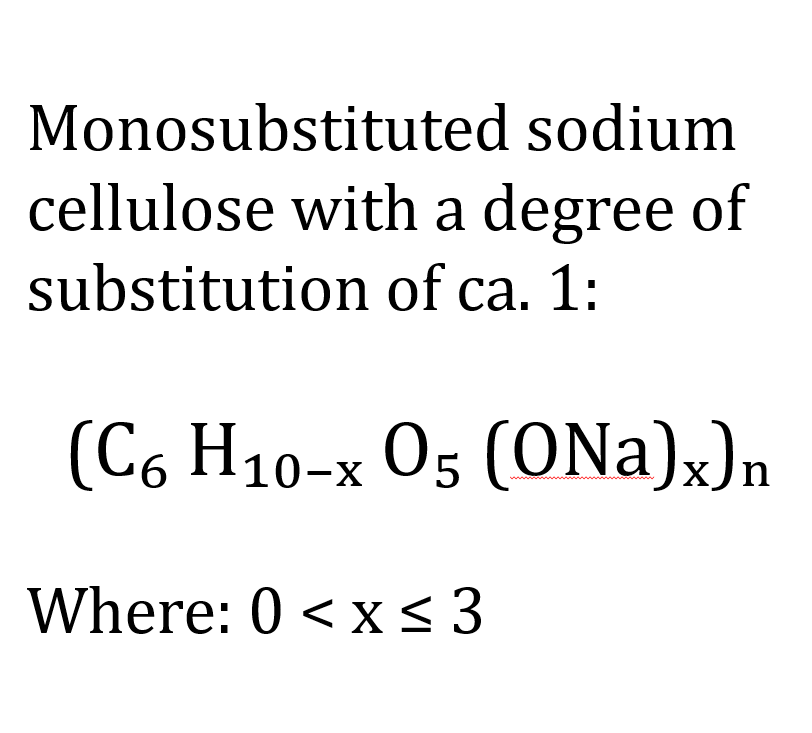
Sodium cellulose
Sodium cellulose is a reactive intermediate product in cellulose chemistry. It is not an end product, but a necessary activation state of cellulose. It is produced when cellulose is treated with concentrated caustic soda. This step is called alkalisation. During this process, the hydroxyl groups of the cellulose are partially deprotonated. This produces cellulose alcoholates in sodium form. The original polymer structure of the cellulose is retained, but becomes significantly more reactive.
Industrially, sodium cellulose is produced from pulp that has been previously processed into a fine powder or fibre material. The caustic soda used often has concentrations of 40 to 50 percent.
Alkalisation is an exothermic reaction that must be temperature-controlled. The resulting product is solid, moist, alkaline and chemically highly reactive. It is highly hygroscopic and sensitive to inhomogeneous wetting. Sodium cellulose itself has no independent economic significance. Its function is to activate the cellulose for subsequent substitution reactions.
Only in this state is it possible to produce cellulose ethers. In the synthesis of carboxymethyl cellulose, sodium cellulose reacts with chloroacetic acid or its sodium salt. This incorporates carboxymethyl groups into the cellulose molecule. Sodium chloride is produced as a by-product. The reaction can be carried out with or without organic solvent as a slurry.
Other cellulose ethers are produced in a similar manner. Gaseous reagents such as methyl chloride, ethylene oxide or propylene oxide are used for this purpose. These processes require elevated temperatures and pressures. Sodium cellulose acts as a nucleophilic reaction centre. The uniformity of the previous alkalisation significantly influences the degree of substitution and its distribution along the polymer chains.
In terms of process engineering, the use of sodium cellulose places high demands on mixing and reactor technology. A homogeneous distribution of the caustic soda in the pulp is crucial. Local over- or under-alkalisation leads to inhomogeneities in the end product. This affects the solubility, viscosity, gel behaviour and application properties of the cellulose ethers. Large-volume mixers and synthesis reactors must therefore ensure uniform wetting, controlled temperature control and gentle treatment of the fibres.

